UNOS News
By Flora Simmons, MD
White Paper Analyzes the Ethical Issues in Normothermic Regional Perfusion
The OPTN board has approved release of a white paper addressing the ethical principles involved in normothermic regional perfusion (NRP), an approach to help facilitate improved organ function after cessation of circulation in donation after circulatory death cases. Ethical concerns have been raised given the involvement of recirculation after declaration of death. This white paper reviews the ethical implications of NRP according to established ethical principles guiding donation and transplantation. Link here
SATA Member Corner: 5 minutes with Dr. Ryan Nazemian from Lahey Hospital and Medical Center
By Alex Ruan, MD
Can you share a little bit about your background and your journey to a career in medicine?
I grew up and went to medical school in Yazd, Iran. Right after medical school, I moved to the United States and found a research position in Cleveland, Ohio, and did my PhD at Case Western Reserve University. My PhD research mentor, James Reynolds, was doing basic science and pharmacology research in anesthesiology. We were working on organ donors and nitric oxide bioactivity. I got introduced to anesthesiology through him, and then I worked with a couple anesthesiologists there. After finishing my PhD, I matched at the anesthesiology program at Case Western Reserve University.
Why transplant anesthesiology?
During residency, I liked the transplant cases. I really like big complex cases. And that made me look into liver transplant fellowship programs and found Lahey here in Burlington, Massachusetts. I came here for fellowship and stayed on ever since.
I really like the complexity of the cases, and the fact that we are very involved with the patient care perioperatively. I think liver transplant is one of the most beautiful procedures that we can do – every case is different and has its own challenges, and once the liver starts working, the patient becomes a completely different person. And of course, given my research background, I really like the research part of it. I think there’s still many, many things that we don’t know about transplant anesthesia and transplant in general. So I think it’s actually a very young field, and there are a lot of opportunities here.
Why did you join SATA? How long have you been a member of SATA and what is your current involvement with the organization?
I found SATA one day during an online search as I was just starting to look into transplant anesthesiology. I found Lahey Clinic through the SATA fellowship database, actually. I’ve really enjoyed being involved with SATA, through it I’ve made a lot of friends, you, Dieter Adelmann, Michael Bokoch, and others that I enjoy collaborating with. I also enjoy going to the meetings – all our meetings are extremely educational and it’s a great opportunity to regularly connect with people. I’m currently part of the Vanguard Committee and lead the Social Media Subcommittee efforts.
What are your research interests, and do you currently have any research/ clinical projects going on?
My research interest has always been the field of transplantation in general. I started with organ donors, but now my research is on outcomes, such as fast-track and ERAS protocols. I am working on the early extubation project that several centers are involved through the SATA database. I also have a project looking at the use of methadone and ketamine for pain management in liver transplantation, which we just presented at ILTS.
What is your favorite piece of anesthesia equipment?
I really like 14g IVs and MAC lines. IVs are such a simple but effective anesthesia instrument. It definitely has saved me many times in the operating room. It’s quite cheap, and so effective. Also, the MAC line – I use it in all my liver transplant cases, It’s relatively easy to place, and overall can be used for many things such as massive blood transfusion
What do you enjoy doing outside of work?
I really enjoy spending time with my family. I have a 2-year-old son, and we are expecting another baby soon. I also love watching movies and sports on TV. I’m a huge fan of FC Barcelona and I watch all their games. I’m hoping that once my son is older, we can go to Barcelona and watch some games together.
What advice would you give to a medical student or resident who is interested in liver transplant?
I encourage everyone to have an open mind on their transplant rotation – some medical students and residents get intimidated by liver transplant because some of these patients are quite sick. I remember I had a resident once and they had a quite challenging month. But then, at the end of the month. I took them to the post-op floor, and we saw some of those patients walking and talking and laughing, which was really amazing.
Dr. Nazemian can be reached at ryan.nazemian@lahey.org or via the SATA Instagram via @transplant.anesthesia
If you or someone you know is interested in being featured in the SATA newsletter, please reach out to Dr. Alexandra Ruan aruan@stanford.edu
In the Spotlight: University of Michigan Medical Center
By David Rosenfeld, MD, FASA
We would like to thank Dr Sathish Kumar, Section Head and Medical Director of Transplant Anesthesiology for University of Michigan, for providing us with the highlights of their well-established program.
Transplant services offered at U of M include adult and pediatric deceased donor and living donor liver transplants, combined liver-kidney and heart-kidney transplants, and a heart-liver transplant program is in the planning stages. Dr. Seth Wait is the Director of liver transplant surgery program.
The adult liver team consists of ten dedicated anesthesia faculty members and a consistent and strong liver anesthesiology fellowship program led by Dr. Sean Ewing. The fellowship has seen an increasing number of external and internal interested candidates, currently with two fellows, including one pursuing a T-32 research track over a two-year period.
The University of Michigan Liver Transplant Program is at the forefront of research and innovation including being the home to multicenter perioperative outcome research and driving clinical trials and studies in liver and kidney transplantation. Currently over 90% of cases utilize normothermic perfusion devices, significantly increasing transplant volumes. Other innovative initiatives include the Rapid Review and Sub-MELD15 Club, Led by Dr. Waits. This initiative helps risk-stratify and accept expedited offers for suitable candidates. They have a unique and focused Liver Transplant Perioperative Cardiac Risk Committee, which is multidisciplinary including anesthesiology, hepatology, transplant surgery, and cardiology experts working to mitigate cardiovascular risks in high-risk transplant recipients. In this group exists a Dedicated Transplant Cardiologist, Dr. Nicole Bhave, who quarterbacks risk stratification for solid organ transplant recipients.
Looking ahead, as the program continues to grow, the University of Michigan Liver Transplant Program is on track to perform its highest transplant volume in program history. They remain committed to providing exceptional care, advancing research, and training the next generation of transplant specialists with a commitment to excellence in patient care, research, and education driving success. Clearly it’s not all football in Ann Arbor and hats off to the great team at Michigan!
Many thanks to Dr. Kumar for sharing details of their program. If interested in having your program highlighted in the future, please contact David Rosenfeld, Mayo Clinic Arizona at Rosenfeld.david@mayo.edu
The Research Corner
By Michael Trostler, MD
Current indications and selection criteria for early liver transplantation in severe alcohol-associated hepatitis. By Ramirez-Cadiz Et Al.
This narrative review of the natural history of alcoholic hepatitis (AH) finds that mortality for alcoholic cirrhosis was decreased with earlier liver transplant. Early transplant without a 6 month period of abstinence from alcohol should be considered as patients with AH may not survive their initial illness, and if they do survive often don’t recover liver function.
Double Blinded RCT performed in South Korea of 205 patients examining the effects of dexmedetomidine (PrecedexⒸ) on the effect of AKI on patients receiving living donor liver transplantation (LDLT). Results were encouraging with 35% of the study group having AKI and 50% of the control group. Lactate levels were also significantly lower in the study group. The potential to reduce ischemia-reperfusion injury during liver transplant hold great clinical importance to morbidity and mortality, this may be one component moving forward to alleviate some of the burden.
Universal intraoperative systemic heparin administration during liver transplantation: A case series By Diaz et al.
This retrospective review at University of Michigan evaluates a change in practice of giving IV heparin prior to portal venous cross-clamp to only selected patients vs all patients. The study only had an incidence of intracardiac thrombus of 1.6%, it may be as high as 5-10%. While the study did not have the power to see a statistical decrease in thrombotic events they did find that total blood product usage did not increase with increasing doses of heparin, and that there wasn’t a higher incidence of bleeding complications. This study suggests that routine use of intraoperative heparin is safe, but notes that further research is needed to find the optimal dose.
Special Topics
By Alex Stoker, MD
Imaging piggyback outflow anastomosis with TEE during orthotopic liver transplantation
Sufficient venous outflow from a transplanted liver is necessary to ensure optimized perfusion pressure and for long term allograft survival. Stenotic piggyback anastomosis results in poor venous outflow and can lead to not only hemodynamic instability due to decreased right ventricular preload but also poor allograft function due to venous congestion. Transesophageal echocardiography (TEE) may be used to visualize the liver outflow anastomosis. Typically, the piggyback anastomosis can be imaged by advancing the TEE probe from the midesophageal bicaval view to image the IVC at the level of hepatic veins. Increasing the omniplane angle anywhere from 30-90 degrees may be necessary to fully visualize the piggyback anastomosis (see figure below). In the case of piggyback anastomosis stenosis, color flow Doppler will reveal flow acceleration with a high velocity and turbulent jet originating at the level of stenosis. It is important to recognize that some flow acceleration may be seen across the anastomosis in the absence of clinically significant stenosis due to a high cardiac output state and hyperdynamic circulation. There is increasing use of TEE to evaluate hepatic and IVC vasculature during liver transplantation and should be considered when venous outflow concern arises (Khurmi et al, 2019).
Reference:
https://pubmed.ncbi.nlm.nih.gov/31321114/
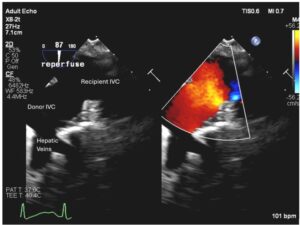
Figure. TEE image with and without color flow Doppler, demonstrating a widely patent piggyback anastomosis after reperfusion of the liver allograft. TEE view obtained by advancing the probe from midesophageal bicaval view to the level of hepatic veins. IVC, inferior vena cava.
SATA Committee and Leadership Updates
The SATA Fellowship Committee – under the leadership of Dr. Ramona Nicolau-Raducu, has been actively involved in various educational initiatives, including a well-received fellow lecture series. Current projects include collaboration with the International Liver Transplant Society on developing guidelines for liver transplant anesthesia fellowships and cultivating networking opportunities for fellows, including planning social events at upcoming liver transplant anesthesia meetings. Our committee contributes to a comprehensive list of liver transplant anesthesia fellows and their directors, and we are committed to enhancing educational programs for fellows. We also continue to work with the Vanguard Committee to connect fellows with speaking opportunities and involve fellows in research presentations at SATA regional meetings. By Christine Nguyen-Buckley, MD
The SATA Membership Committee – was initially responsible for establishing the SATA membership list and membership communications. After this foundational work, we have been able to grow SATA with the expert support of PACA, our management company. While PACA now handles day-to-day membership operations, the SATA Membership Committee remains dedicated to addressing any member concerns. We are committed to both recruiting new members and retaining our valued current members. Our past initiatives have been aimed at enhancing the value of SATA membership. We also continue to partner closely with other committees such as the Vanguard and Fellowship committees. We invite you to share your thoughts on how we can further enhance the SATA membership experience by reaching out to us at sata@pacainc.com. By Christine Nguyen-Buckley, MD
Abdominal Transplant Committee – for SATA has been quite busy over the past 3 years. We are excited to share that we have published two white papers in Clinical Transplantation. “Regional Anesthesia for Transplantation Surgery – A White Paper Part 1: Thoracic Transplantation Surgery” was published in June 2023. The subsequent installment entitled, “Regional Anesthesia for Transplantation Surgery – A White Paper Part 2: Abdominal Transplantation Surgery” was published in January 2024.
Since this recent success, the members of the ATC have started work on two new projects. We are currently in the literature review stage for a planned review paper covering machine perfusion in liver transplantation surgeries. The group is also in the beginning stages of a survey-based project that will poll anesthesia liver transplant directors regarding the use of regional anesthesia in the perioperative setting of liver transplantation surgeries.
The ATC hopes to attract additional SATA members who provide care for abdominal transplantation patients at their home institutions. We are looking for members who have an interest in research as well as further refining anesthesia care in the field of abdominal transplantation surgeries. If interested in joining SATA’s Abdominal Transplant Committee, please reach out to any ATC members. By Michael Ander, MD
SATA Seed Grant Application
We invite you to submit your application for the 2025 Seed Grant! The SATA Seed Grant is a one-year, $5,000 starter grant for transplant projects, open to junior faculty members and trainee physicians (residents and fellows). This grant aims to inspire and assist aspiring faculty/trainee physicians in initiating a transplant-related research project. It is specifically intended for projects that have not previously received extramural / non-departmental funding. Recipients must have sufficient departmental support to complete the project within one year.
Application/Grant Cycle Timeline
| October 11, 2024 | Announcement of the grant |
| November 4 – December 2, 2024 | Proposal Submission (Phase 1) |
| December 18, 2024 | Invitations to submit full proposals will be sent out |
| January 20, 2025 | Submission deadline for full proposals (Phase 2) |
| February 19, 2025 | Announcement of the awardee and send letters of feedback to the other applicants |
| March 23, 2025 | Project presentation at SATA National Meeting in Honolulu, HI |
| July 1, 2025 | Grant initiation |
| December 2025 | Submission of the mid-term report |
| August 2026 | Submission of the final report |
Eligibility Requirements
Applicants must:
- Be a current member of the SATA.
- Have received no prior extramural (“outside”) research funding for this specific project.
- Be a trainee (resident/fellow) with dedicated research time or faculty within ten years of their first appointment.
- Present an original project idea.
- Name a mentor and submit a mentoring plan (only required for Trainees and Faculty within three years of their initial appointment)
- Have the full support of the Department Chair, who must sign off on the grant application if awarded.
- Have the full support of the Program Director if the applicant is a trainee.
- Submit a budget plan. This grant does not cover overhead or salaries, as it is a starter grant. While the SATA Seed Grant cannot be applied to salary support, it can be used to support costs for professional services from salaried professionals that are essential for the planning or execution of the project (e.g., statistician fees).
- Agree to present the completed study (or a progress report) at the SATA National Meeting following the award, including a financial report detailing how the grant was used.
- When submitting a manuscript to a peer-reviewed journal for publication, the investigators must acknowledge SATA Seed Grant funding.
Application requirements, instructions, and process.
The grant application must include the following and must be submitted to sata@pacainc.com:
Phase 1:
- Letter of Intent
To include:
- Project Title
- Name of the Investigator & Mentor
- Description of the Proposed Research (500-word limit).
Structure:- Background & Significance (2 paragraphs)
- Specific Aim (1 paragraph)
- Methods (1-2 paragraphs)
- Applicant Biosketch
- (NIH Biosketch), including a personal statement tailored to this application.
Phase 2:
The SATA Research Committee will select three submissions and invite the three applicants to submit a complete application & a 5-minute recorded presentation of their planned research.
Additional Documents for Phase 2:
- Detailed project plan
To include Objective, Background, Hypothesis, Aims, Methods and Materials, Timeline, Pitfalls, Solutions, Significance, and References. (2 pages + references) - Mentor Biosketch
(A mentor is required if the PI is a trainee or a junior faculty member – within three years of their initial appointment) - Budget and Budget Justification
Please note: the grant does not cover overhead or investigator salaries. (1 page) - Mentoring plan
A mentoring plan, written by the mentor, is required if the PI is a trainee or a junior faculty member – within three years of their initial appointment (1 page) - Letter of support and commitment
From the Mentor (if the PI is a trainee or a junior faculty member – within three years of their faculty appointment) or the Department Chair (for faculty members starting in the 4th year of their appointment) (1 page)
Selection Process
The review process will consist of two phases: The SATA Research Committee & SATA Council will review all letters of intent (Phase 1). Three investigators will be invited to submit a full proposal (Phase 2). One grant will be awarded per year.
Reporting and Award Requirements
Awardees must submit two progress reports six months and one year after the receipt of the grant:
The 6-month report should be brief (less than two pages) and include:
- Progress made to date (including expenses).
- Difficulties encountered or anticipated roadblocks and plans to mitigate them.
- Identification and explanation of any changes made from the original proposal.
- The committee will review the progress report and help resolve problems that arise to ensure the success of the grant recipient.
The 1-year report must be provided for review 13 months after the beginning of the Grant Period. The report will include:
- A summary of the objective and the results of the study,
- Any changes in the research project or mentorship,
- Publication or abstracts that have been generated from the study,
- A financial report detailing how the grant money was spent,
- Award of further funding.
The grant will be awarded to the grant recipient’s institution. All expenses funded by the grant are to be paid by the institution. Any unused funds are to be returned to SATA. The SATA and its auditors reserve the right to receive documentation and itemized expense receipts upon request.
Please address your questions regarding the application process to Dr. Dieter Adelmann (Chair of the SATA Research Committee) at dieter.adelmann@ucsf.edu.
For administrative questions, please reach out to the SATA Office at sata@pacainc.com.
Upcoming Events and Meetings
SATA Meetings:
SATA Southeastern Meeting, Virtual
Saturday November 16, 2024
SATA West Annual Meeting, San Francisco, CA
Saturday December 14th, 2024
The 2025 Symposium for the Society for the Advancement of Transplant Anesthesia
SATA Annual Meeting, Honolulu, HI
Monday March 23, 2024
(In conjunction with the IARS Annual Meeting)
More information coming soon!
Other Meetings:
KoreAnesthesia, Korean Society of Anesthesiologists Annual Meeting
November 7-9, 2024, Incheon, Korea
The Liver Meeting, The American Association for the Study of Liver Disease (AASLD)
San Diego, CA, November 15, 2024
The International Society for Heart and Lung Transplantation (ISHLT) Annual Meeting
Boston, MA, April 27-30
UNOS Transplant Management Forum
San Antonia, TX, May 5-7, 2025
The International Liver Transplant Society (ILTS) Annual Congress
Singapore, May 28-31, 2025
SATA BOARD OF DIRECTORS: TERM 2023 – 2024
President
Gebhard Wagener, MD
Immediate Past President
Tetsuro Sakai, MD, PhD, MHA, FASA
Founding President
Ernesto A. Pretto Jr., MD, MPH
President-elect
Lorenzo De Marchi, MD
Secretary
Jiapeng Huang, MD, PhD, FASA, FASE
Treasurer
Ranjit Deshpande, MBBS
Executive Council
Michael Ander, MD, FASA
Adrian Hendrickse, BM, MMEd, MAcadMEd, FRCA
Sathish S. Kumar, MD
Raymond M. Planinsic, MD, FASA
Newsletter Editor-in-Chief
David Rosenfeld, MD, FASA
Newsletter Editorial Board
Sennaraj Balasubramanian, MD
Amit Bardia, MD
Andrew Gorlin, MD
Jiapeng Huang, MD, PhD, FASA, FASE
- Susan Mandell, MD, PhD
Sergio Navarrete, DO
Yong G Peng MD, PhD
Alexandra Ruan, MD
Flora Simmons, MD
Natalie Smith, MD
Alex Stoker, MD
Michael Trostler MS, MD