T. Everett Jones, MD, and Yong G. Peng, MD, PhD, FASE, FASA
Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL
The learning objectives of this PBLD are to:
1) Define the essential evaluation of patient comorbidities before left ventricular assist device (LVAD) placement.
2) Review the impact/implications of a previous MitraClip procedure for patients undergoing LVAD placement.
3) Identify the pertinent transesophageal echocardiography (TEE) findings relevant to LVAD placement.
4) Discuss the strategic approach and management of patients undergoing LVAD placement.
Case Report
A 49-year-old man had a past medical history of ischemic cardiomyopathy, coronary artery bypass grafting 10 years prior, chronic renal insufficiency, obesity (BMI 36), congestive heart failure, left ventricular ejection fraction (LVEF) of 20% to 25%, previous St. Jude pacer/automatic implantable cardioverter defibrillator (AICD) for primary prevention, and severe mitral regurgitation after a MitraClip procedure (Abbott, Abbott Park, IL, USA). He presented for placement of a HeartMate III left ventricular assist device (LVAD) as destination therapy. A pre-induction radial arterial line was placed. General endotracheal anesthesia was induced with midazolam, fentanyl, lidocaine, propofol, and rocuronium. The right internal jugular vein was then cannulated using ultrasound guidance with an introducer and a pulmonary artery catheter was floated without incident. His AICD was turned off before the procedure began. Transesophageal echocardiography (TEE) revealed a dilated left ventricle (LV) with global severe hypokinesis and a LVEF of approximately 20%, along with grossly normal right ventricular (RV) function, severe mitral regurgitation, and mild tricuspid regurgitation. A MitraClip was visualized without evidence of mitral stenosis (mean gradient 2 mmHg; Figure 1A and B). A possible residual atrial septal defect (ASD) was identified by color-flow Doppler examination of the interatrial septum. The patient underwent redo sternotomy and was cannulated for cardiopulmonary bypass (CPB). While on CPB, an iatrogenic ASD resulting from his previous MitraClip procedure was closed, the Heartmate III LVAD inflow cannula was placed in the apex of the LV, and the outflow cannula was placed in the ascending aorta. While examining the patient with TEE while he was on CPB, we noted that the inflow cannula was not optimally aligned (Figure 2). The surgeon adjusted the inflow cannula to allow for a more appropriate flow. The LVAD speed was slowly increased in increments of 200 RPMs while the patient was weaned from CPB. A pump speed of approximately 5100 RPMs was eventually reached. The patient was successfully weaned from CPB and protamine was administered. The total CPB time was 147 min. The patient was successfully transitioned to LVAD support without difficulty on infusions of milrinone, epinephrine, norepinephrine, and 40 ppm inhalational nitric oxide (NO). Inflow cannula peak velocities measured between 80 and 100 cm/s on TEE. The patient was transported to the cardiac intensive care unit uneventfully. He was extubated on postoperative day 2.
Discussion
1. Why is preoperative evaluation of patient comorbidities important for LVAD placement?
Preoperative evaluation is vital for ventricular assist device placement. Discussions with the surgeon about the urgency of placement (elective vs emergent), planned surgical approach (sternotomy vs thoracotomy), and likelihood of RV assist device placement, as well as any additional planned surgical procedures is necessary. It is important to determine the etiology of the patient’s cardiac and coexisting diseases, paying particular attention to the patient’s current medications, inotropic infusions, pulmonary artery pressure, and RV function. The presence or absence of an AICD and the management plan for the AICD should be noted. Additionally, take note of the hematocrit; many patients have received erythropoietin stimulating agents preoperatively and removal of autologous blood may be considered.
2. What pertinent preprocedural TEE findings are relevant for LVAD placement?
A systematic TEE examination is essential before LVAD placement, paying particular attention to the assessment of structural and functional abnormalities. Significantly reduced LV contractile function in this population increases the risk of LV thrombus. The presence of a intracardiac shunt such as a patent foramen ovale (PFO), ASD, or ventricular septal defect (VSD) can lead to hypoxia after LVAD placement secondary to right to left shunting. Structural valvular pathologies such as aortic regurgitation or mitral stenosis will negatively affect the function of the LVAD. Close to normal RV systolic function is essential for the success of LVAD placement.
3. How can a history of a MitraClip procedure affect LVAD placement?
The MitraClip procedure is a structural heart intervention that attempts to address severe mitral regurgitation in patients whose comorbidities prohibit an open mitral valve repair or replacement. The procedure requires a trans-septal puncture through the interatrial septum to deliver the mitral clip. The intervention is successful when a significant reduction of mitral regurgitation is achieved without causing mitral stenosis. However, in certain circumstances, mitral clip application may result in mitral stenosis and/or no significant reduction of mitral regurgitation. The ASD (Figure 3) is typically not closed at the conclusion of the procedure. This iatrogenic intracardiac shunt typically closes spontaneously over time, but this is not always the case. If it is present in a patient undergoing LVAD implantation, it is necessary to close the defect.
4. How can TEE guide LVAD inflow and outflow cannula placement?
The preoperative TEE examination should rule out LV thrombus, and intraoperatively it can guide the surgeon to locate an appropriate area to place the inflow cannula at the LV apex. The goal is to directly align the inflow cannula with the mitral valve inflow. The LVAD inflow cannula should avoid angulation toward either the septal wall or the lateral wall. The LVAD inflow cannula velocity should be laminar and approximately 100 cm/s. The LVAD outflow cannula is located in the proximal ascending aorta. TEE assessment should be free of aneurysm, dissection, or significant atheromatous disease at the site of LVAD outflow cannula placement.
5. Should separation from CPB be accomplished by setting the LVAD to full speed immediately?
LVAD devices require constant hemodynamic and echocardiographic monitoring in the immediate post-implantation period during and after weaning from CPB. LVAD function is preload dependent and afterload sensitive. After separation from CPB, ensuring adequate fluid replacement and making slow adjustments to the LVAD speed are strongly recommended. Failure to maintain normovolemia and adequate LV filling can lead to “suction events” (Figure 4), which can lead to acute right heart failure due to changes in the RV geometry. The medical professional should be aware of the potential for suction events and rely on institutional/peer-reviewed protocols to prevent such devastating yet avoidable events.
6. Why is monitoring RV systolic function essential during placement of a LVAD?
Many patients undergoing LVAD placement present with pulmonary arterial hypertension with tenuous RV systolic function. It is essential to constantly monitor RV systolic function during the course of LVAD placement. Particular attention should be paid to avoiding increases in pulmonary vascular resistance. Thus, it is necessary to avoid hypoxia, hypercarbia, significant sympathetic stimulation, hypothermia, and acidosis; these factors may result in an acute increase in pulmonary vascular resistance. Administration of inotropic agents such as milrinone and dobutamine, as well as pulmonary vasodilators such as inhaled nitric oxide or epoprostenol can be helpful to ensure that the RV systolic performance can provide the LV filling necessary to keep up with the increased LV output from LVAD function.
7. What is the pulsatility index?
The pulsatility index (PI) is a measure of the pulse flow through the LVAD device from native heart function. LV contraction leads to an increase in ventricular pressure that increases pump flow; the flow pulses generated are measured and averaged over 15 s. The PI increases with enhancement of LV ejection. The PI can abruptly drop due to a sudden decrease of LV output resulting from arrhythmias or LV preload. Barring a decrease in circulating volume, acute RV failure can also lead to a drop in the PI.
8. How should fluid resuscitation and vasoactive support be balanced immediately after LVAD implantation?
TEE is an invaluable modality to guide fluid management after LVAD placement. Appropriate LVAD function demands primary fluid replacement instead of continued increases in vasoactive medications. Echocardiography can not only assess the appropriate alignment of the LVAD inflow cannula at the LV apex, but can also be used to ensure adequate filling of the LV. Hypovolemia and acute RV failure both lead to inadequate LV filling that can result in “suction events.” These events can precipitate acute right heart failure or worsen preexisting right heart failure. TEE is useful to evaluate RV function after LVAD implantation, and if RV function is adequate, then volume replacement should be considered. LVAD devices are afterload sensitive and preload dependent. Therefore, vasoconstrictors should not be the primary choice to rescue hypotension; rather, consideration of volume replacement is warranted. Inotropic agents such as epinephrine, milrinone, or dobutamine should be administered to support RV systolic function to promote LV filling. This strategy will allow for appropriate performance of the LVAD device.
References
- Silverton NA, Patel R, Zimmerman J, et al. Intraoperative transesophageal echocardiography and right ventricular failure after left ventricular assist device implantation. J Cardiothorac Vasc Anesth. 2018;32;2096–2103.
- Gudejko MD, Gebhardt BR, Zahedi F, et al. Intraoperative hemodynamic and echocardiographic measurements associated with severe right ventricular failure after left ventricular assist device implantation. Anesth Analg. 2019;128;1:25–32.
- Baxter RD, Tecson KM, Still S, et al. Predictors and impact of right heart failure severity following left ventricular assist device implantation. J Thorac Dis. 2019;11;6:S864–S870.
- Rich JD, Burkhoff D. HVAD flow waveform morphologies: theoretical foundation and implications for clinical practice. ASAIO J. 2017;63:526–535.
- Katz WE, Conrad Smith AJ, Crock FW, Cavalcante JL. Echocardiographic evaluation and guidance for MitraClip procedure. Cardiovasc Diagn Ther. 2017;7:616–632.
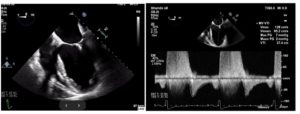
Figure 1. (A) Mid-esophageal four-chamber view reveals the mitral clip between the anterior and posterior mitral valve. (B) Pulsed-wave Doppler reveals a mitral inflow pattern with a peak gradient of 7 mm Hg and mean gradient of 2 mm Hg.
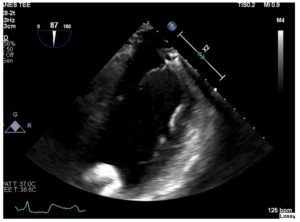
Figure 2. Mid-esophageal two-chamber view reveals malalignment of the LVAD inflow cannula.
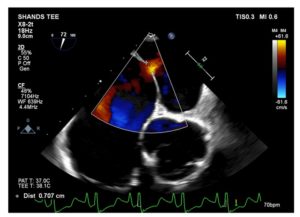
Figure 3. Modified mid-esophageal aortic short-axis view reveals classic example of residual ASD after the MitraClip procedure.
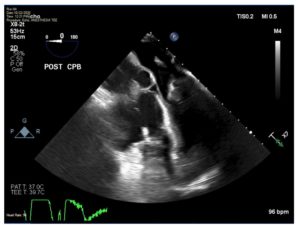
Figure 4. Mid-esophageal four-chamber view demonstrates severe hypovolemia with an obliterated left ventricle.